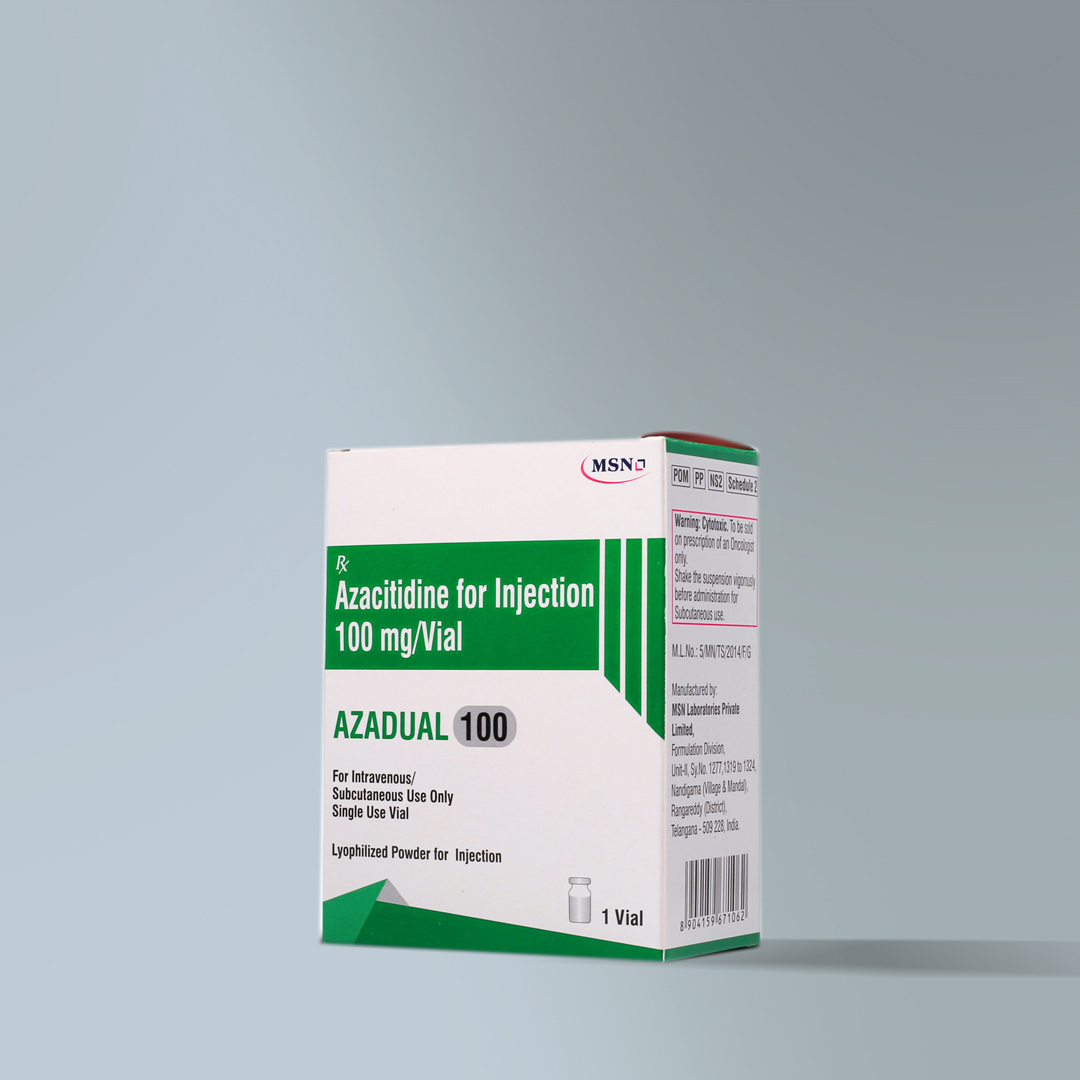
Azacitidine (Azadual)
Azacitidine is believed to exert its antineoplastic effects by multiple mechanisms including cytotoxicity on abnormal haematopoietic cells in the bone marrow and hypomethylation of DNA.
Share Now
Additional Information
| Name | Azacitidine |
| Description | Azacitidine is believed to exert its antineoplastic effects by multiple mechanisms including cytotoxicity on abnormal haematopoietic cells in the bone marrow and hypomethylation of DNA. The cytotoxic effects of azacitidine may result from multiple mechanisms, including inhibition of DNA, RNA and protein synthesis, incorporation into RNA and DNA, and activation of DNA damage pathways. Non-proliferating cells are relatively insensitive to azacitidine. Incorporation of azacitidine into DNA results in the inactivation of DNA methyltransferases, leading to hypomethylation of DNA. DNA hypomethylation of aberrantly methylated genes involved in normal cell cycle regulation, differentiation and death pathways may result in gene re-expression and restoration of cancer-suppressing functions to cancer cells. The relative importance of DNA hypomethylation versus cytotoxicity or other activities of azacitidine to clinical outcomes has not been established. |
| Active Ingredient | Azacitidine |
| Indication | Azacitidine Accord is indicated for the treatment of adult patients who are not eligible for haematopoietic stem cell transplantation (HSCT) with: – Intermediate-2 and high-risk myelodysplastic syndromes (MDS) according to the International Prognostic Scoring System (IPSS), – chronic myelomonocytic leukaemia (CMML) with 10-29 % marrow blasts without myeloproliferative disorder, – acute myeloid leukaemia (AML) with 20-30 % blasts and multi-lineage dysplasia, according to World Health Organisation (WHO) classification, – AML with >30% marrow blasts according to the WHO classification. |
| Strengths | 100 mg/mL |
Related Products
Related products
Golexin (Goserelin) LA 10.8 mg
Rated 0 out of 5ARSENIC TRIOXIDE
Rated 0 out of 5Aploda (Capecitabine) 150mg & 500mg
Rated 0 out of 5Onda 8mg/4mg Solution For Injection Ampoule (Ondansetron)
Rated 0 out of 5
Disclaimer
Pharma Solution is the marketing agent and Distributor for the listed products for the Gulf and MENA region. The products are either registered and approved by the respective local health authority or contracted by the MAH to supply to the end users on a named patient basis. Some products such as Orphan drugs, rare diseases medicines are imported based on special import permits and as per need of the Hospitals in compliance with full documentation.